Ethics and Personal Data Management
The study is implemented in full compliance with all the principles of Ethics in Biomedical Sciences. More specifically, patient enrolment in the study was initiated following the research protocol approval by the Bioethics Committee of the NKUA, Nursing Department and the Scientific Committees of the study centres/hospitals.
Prior to the study commencement, the Centers’ field researchers were specifically trained on the General Principles of Good Clinical Practice and patients were briefed in detail about the purpose of the study and their rights (e.g. the right to volunteer, the right to refuse or cease their participation at any stage of the study), and their written informed consent to participate in the study was sought after (by themselves or their relatives/companions).
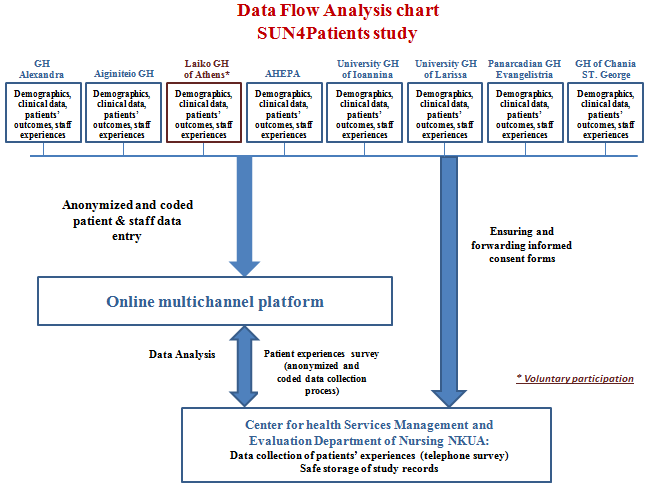
In addition, taking into consideration the recent implementation of the General Regulation (EU) 2016/679 on Data Protection (GDPR), Article 6 and Article 9, and being fully aware of the importance of protecting participants’ personal data and avoiding any breach of their personal data, a strict policy regarding confidentiality and personal data management policy is applied. Data is anonymized and encoded in a manner that the patients' identity is not disclosed in any way. Data flow is depicted in this Diagram.